
But the developmental paths of the major COVID-19 vaccines suggest that future vaccines can achieve a radically shortened time to market if there are many candidates and modalities to work with. There are no safe bets in vaccine development-and our analysis shows that investments have abated following the surge of capital during the initial COVID-19 crisis, in 2020–21. For more, see “Fact sheet: President Biden to launch a National Biotechnology and Biomanufacturing Initiative,” The White House, September 12, 2022. 4 The United States has launched an initiative focused on manufacturing in biotechnology.

The US government, for example, recently launched an initiative aimed at ensuring that biotechnology manufacturing capabilities are available in the country.
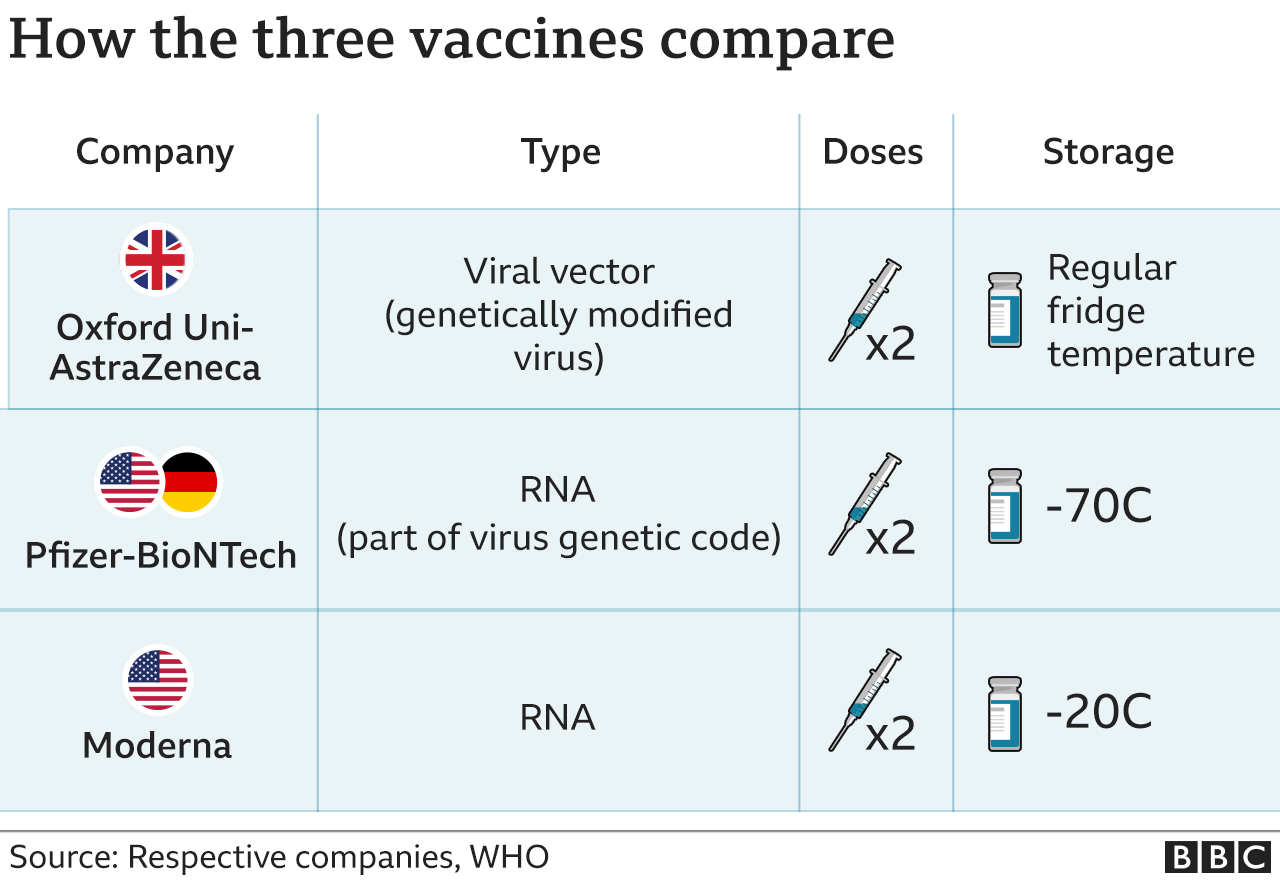
This suggests that creating more resilient vaccine ecosystems-ones that allow a country or region to develop and trade expertise and resources and collaborate with partners to secure enough vaccine doses when they need them-is likely to play a critical role in readiness in the future. shifting expectations of what is possible in development and manufacturing and highlighting immunization as a viable path to protect populations quickly. Offodile II, “Closing the global vaccine equity gap: Equitably distributed manufacturing,” Lancet, May 21, 2022, Volume 399, Number 10339.

COVID-19 laid bare the gaps in the global vaccine supply, 3 Celynne A. Preparing for the next pandemic is a priority for many national public-health leaders and requires them to lay the groundwork to mount an effective vaccine response. 2 “WHO director-general declares the ongoing monkeypox outbreak a Public Health Emergency of International Concern,” WHO, Jnote: WHO is actively working with partners and experts around the world to rename monkeypox. the monkeypox outbreak has been declared a public-health emergency of international concern by the World Health Organization (WHO). Even as COVID-19 enters the endemic stage in the United States and Europe, 1 “ When will the COVID-19 pandemic end?,” McKinsey, July 28, 2022. I bet you every day they run into some vaccine challenge and every day they solve it, and that goes into their playbook,” he said.Public-health crises have vaulted to the top of national agendas over the past two years.

“Nobody’s ever produced mRNA vaccines at this scale, so you can bet your bottom dollar the manufacturers are learning as they go. The increased speed and capacity is not unexpected, said Robert Van Exan, president of Immunization Policy and Knowledge Translation, a vaccine production consulting firm. "Just in the last month we've doubled output." "We call this 'Project Light Speed,' and it's called that for a reason," said Chaz Calitri, Pfizer's vice president for operations for sterile injectables, who runs the company's plant in Kalamazoo, Michigan. Pfizer expects to nearly cut in half the amount of time it takes to produce a batch of COVID-19 vaccine from 110 days to an average of 60 as it makes the process more efficient and production is built out, the company told USA TODAY.Īs the nation revs up its vaccination programs, the increase could help relieve bottlenecks caused by vaccine shortages. Watch Video: How the new RNA technology is used to create the COVID-19 vaccines


 0 kommentar(er)
0 kommentar(er)
